Lithium-ion Car Battery Recycling Advisory Group
Background Information
Provided in Preparation for Meeting #1
Nov. 18, 2019
Mission Statement
This document represents work performed by staff of the Department of Toxic Substances Control (DTSC) and the Department of Resources Recycling and Recovery (CalRecycle) during Summer and Fall 2019, intended to provide a background to the science, uses, and regulations related to the disposal of lithium-ion batteries, especially those used in electric vehicles. The purpose of this background is to educate and inform the reader, who will serve on the Secretary of the California Environmental Protection Agency’s (CalEPA) Lithium-Ion Car Battery Recycling Advisory Group. This document is not meant to be comprehensive.
The Advisory Group is being convened in accordance with Assembly Bill No. 2832 (Dahle, Statutes of 2018). AB 2832 requires the Secretary for Environmental Protection to appoint and convene a Lithium-Ion Car Battery Recycling Advisory Group to advise the Legislature on policies pertaining to the recovery and recycling of lithium-ion vehicle batteries sold with motor vehicles in the state. In addition to members of the environmental community, auto dismantlers, public and private representatives involved in the manufacturing, collection, processing and recycling of electric vehicle batteries, and other interested parties, DTSC, CalRecycle, and CalEPA will each also send a representative to serve on the Advisory Group.
Composition of EV lithium-ion batteries
Battery performance requirements are dependent on the intended application of said battery. In particular, for electric vehicles (EVs), there are two battery characteristics of importance: energy, which can be roughly translated as vehicular driving range, and power, which can be roughly translated as vehicular acceleration. Unlike batteries for hybrid electric vehicles (HEVs) or partial hybrid electric vehicles (PHEVs), batteries for EVs need more energy capacity (for the longer driving distances which would not be supplemented by traditional internal combustion engine),
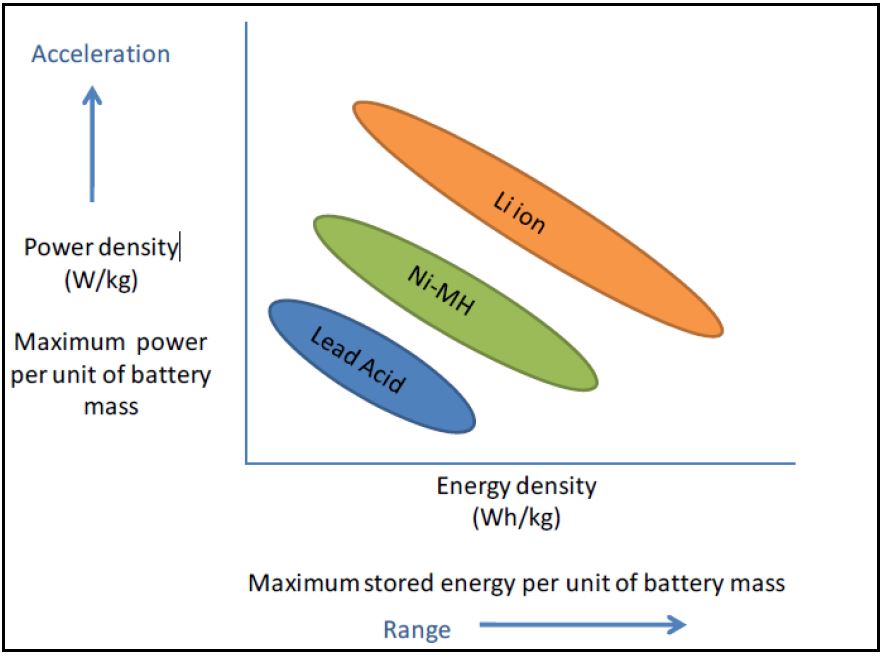
Figure 1 – Power v Energy Comparison of Battery Technologies
as well as high power density for acceleration purposes. Cycle durability (i.e., the number of times a battery can be fully charged and discharged) is also important. These factors inform the preferability of lithium-ion batteries (LIBs) as the most suitable existing technology for EV use. Energy density can also be improved in lithium-ion batteries (LIB), as compared to nickel cadmium (Ni-Cd) and nickel metal hydride (NiMH), making them the preferred battery type for EV use (Gereffi et al). LIBs are used in PHEVs and EVs and have a primary lifetime of 8-10 years. NiMH batteries have about 80% of the market share for HEVs and have a primary lifetime of about 15 years.
A LIB is a rechargeable battery, in which lithium ions move from anode to cathode during discharge. The cathode emits lithium ions to the anode during the charge cycle. During the discharge cycle, the lithium in the anode is ionized and emitted to the electrolyte; the ions move through a porous plastic separator and insert into cathode. Simultaneously, electrons are released from the anode, creating an electricity flow that is then used to power the EV. The anode is composed of carbon (i.e., graphite powder); the cathode of a lithium metal oxide powder; the electrolyte of lithium salts and organic solvents; and the separator of plastic (i.e., polypropylene or polyethylene) and micro-porous membranes (Gereffi et al). Generally, the lithium metal oxide in the cathode will include titanium, nickel, cobalt, manganese, and/or aluminum (Richa).
Figure 2 – Lithium-ion battery cell and module
LIBs are sold in “battery packs,”, which are composed of LIB cells placed into LIB modules and include battery management systems to prevent short-circuiting.
LIBs also have internal safety structures to address concerns related to internal pressure and overcurrent. Overcurrent is any current load in excess of the safety rating of the equipment or the ampacity of a conductor. For example, LIBs have tear-away tabs to reduce internal pressure, safety vents for air pressure relief, and thermal interrupters, called positive temperature coefficient thermistors, for overcurrent protection (Gereffi et al). Additionally, some battery companies insert a metal center pin as a pillar to strengthen against bending force, and to put insulators on the edge of the electrode, where short circuit accidents are most likely to generate.
Economics of Lithium-ion Batteries
In the year 2013, the consumer electronics drove the global revenue-based demand for LIBs with a market share of 60% (Frost & Sullivan). However, LIBs have become the preferred battery system for commercial EVs, starting with the Tesla Roadster and continuing with models from leading manufacturers, such as Chevrolet, Honda, Nissan, Ford, and others. The United States Energy Information Administration (EIA) has predicted widespread diffusion of EVs in the future in the US, with a range of anywhere from 449,000 to 1.146 million EVs sold annually in the US by the year 2020 (EIA), while Deutsche Bank has forecasted up to 4 million EVs sold annually in the US by 2020. Internationally, the number of EVs sold annually is forecasted to range from 5.2 million to 19.8 million (J.D. Power, International Energy Agency, Credit Suisse, and Deutsche Bank).

Figure 3 – Lithium-ion Battery Pack
While these forecasts vary across agencies and parameters (such as economic growth, the price of oil, political pressures, battery technology advancements, etc.), the conclusion is clear – a large-scale deployment of LIBs will be necessary to power these EVs.
That said, the high cost of LIBs for EVs is a concern that must be addressed. LIBs are four to eight times more expensive than lead acid batteries, and one to four times that of NiMH (Nishino).
The recycling value of NiMH batteries is driven by the price of nickel, while the recycling value of LIBs is driven by the price of cobalt.
Recycling Technologies and Entities
The tradeoffs between recycling processes are highly relevant to scale-up: While hydrometallurgy recovers a greater fraction of battery materials, pyrometallurgy may prove to be preferable in the face of small battery volumes and uncertain technologies because of its ability to accept a wide range of chemistries, including both lithium-ion (Li-Ion) and nickel-metal hydride (NiMH) batteries.
Recycling Methodologies for Lithium
- Pyrometallurgical recycling – Lithium-ion battery recycling process that uses smelting to recover precious metals and slag
- Hydrometallurgical recycling – Lithium-ion battery recycling process that uses chemical leaching to extract materials
- Pyrometallurgy is a flexible process, capable of accepting a wide variety of LIB chemistries in a single facility, as well as nickel metal hydride (NiMH) batteries, that focuses on recovering only high-value materials including nickel, cobalt, and copper; unrecovered materials are incorporated into slag that can be sold as a cement supplement
- Hydrometallurgy is a more specialized, chemistry-specific process capable of recovering lithium and aluminum in addition to higher- value metals.
Companies in North America that Recycle EV Batteries
- Retriev – Li-ion and NiMH; Anaheim, CA (HQ); Lancaster, OH (recycling facility)
o Awarded a $9.5 million grant from the US DOE in 2014 for Li-ion recycling - Inmetco – NiMH; Pittsburgh, PA
- Glencore/Xstrata –NiMH and Li-ion; Switzerland (HQ), US locations, Quebec (smelter)
- Umicore – Li-ion and NiMH batteries; Belgium (HQ), Maxton, NC (NiMH treatment and dismantling)
- RMC – they recycle NiMH. Ontario, Canada
Barriers and Incentives
Barriers
- Recycling not cost-feasible at scale
- Difficult to recover all materials in the battery – currently research and pilot projects to improve methods and increase recovery
- Lack of battery pack standardization, different chemistries and cell structure make recycling hand to automate, requires costly manual disassembly
- Battery chemistry likely to change significantly in next decade. Potential changes are low cobalt or cobalt free cathodes (with higher nickel content), which makes recycling even less profitable to recycle since cobalt is the most profitable recovered product
- Collection and subsequent transportation to recycling facility difficult and costly
Additional barriers to repurposing for storage
- Lack of batteries standardization; no standard way to test battery life
- Performance inconsistency for batteries that are old and outdated technologies
- Not included in the EU Batteries Directive, once batteries certified as waste, cannot be put back into circulation without auditable trail of tests (EU)
Incentives
- Supply of cobalt dependent on a few mines in the Democratic Republic of Congo, a politically unstable county, and EV market growth expected to push beyond current supply chain. Battery manufacturers interested in decreasing cobalt content of batteries and a reliable supply of that resource.
- Emerging industry, opportunity for profit given expected EV market growth, so many parties interested including metal miners and refiners, lithium battery manufacturers, lead-acid battery manufacturers, and consumer electronics recyclers.
Potential Environmental and Human Health Impacts
According to the U.S. Department of Energy (DOE), between 295,000 to 1.423 million battery packs will be discarded in the U.S. by 2020 (Chaitanya). These estimates are predicated on a lifetime of five years to 10 years. A second estimate suggests that the LIB waste stream in 2020 could contain approximately 3,400 metric tons of lithium-ion cells just from EV applications, with a baseline estimate of 1.3 million tons generated cumulatively between 2015 and 2040 (Richa). A LIB is considered to have reached its end-of-life when it can no longer provide 80% of its energy (i.e. range) or 80% of its peak power (i.e. acceleration). It is estimated that even after the end of their useful life in EVs, LIBs retain about 70-80% of their capacity, thus being capable of serving less demanding energy storage functions in the utility sector (Richa: Heymans et al., 2014, Williams and Lipman, 2010; Neubauer et al., 2012; Neubauer & Pesaran, 2011; Cready et al., 2003, Narula et al., 2011). For example, used LIBs can be used for EV charging, energy storage (including of solar energy), and street lighting (Stringer 2018). End of life LIBs can then continue in this secondary capacity for an additional seven to ten years after being removed from the original EVs. California is likely to reach a battery pack disposal rate sufficient to justify a pilot-scale facility running at full capacity by approximately 2030 (see Figure 4) and the California Energy Commission’s document: “Plug-in Electric Vehicle Battery Recycling Scale-Up Strategies for California 2015-2050.”
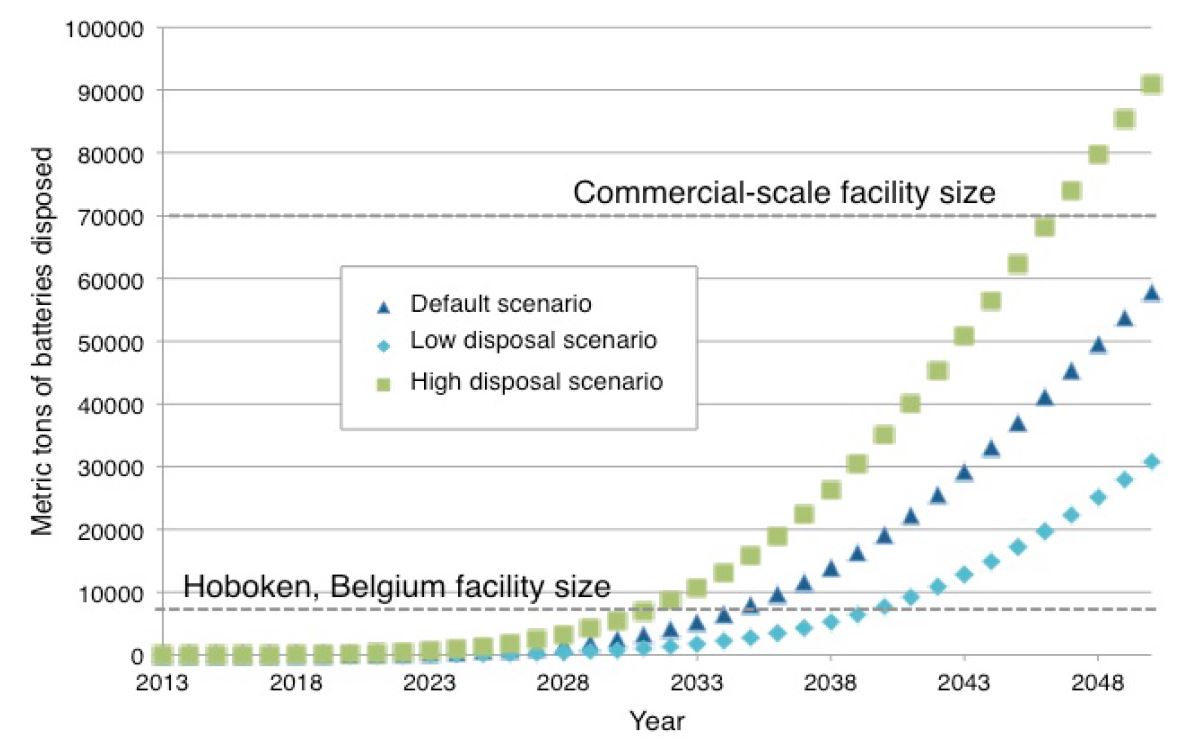
Figure 4 – Mass of Lithium-ion Batteries Available for Recycling by Year (CA)
Based on the above estimates for initial and secondary LIB lifetimes, anywhere between twelve and twenty years after the initial production of the LIB for its use in an EV, the LIB would then enter the waste stream in some fashion. The material-specific composition of the LIB component of the waste stream is expected to remain constant – about 30% metals (lithium, nickel, iron, cobalt, aluminum, copper, and manganese); 25% carbon (e.g., graphite); 22% steel; and 23% plastics, binders, electrolytes, and other non-metals (Richa). Of these constituents, only the metals, including steel, would be expected to be recycled due to the existence of an existing market, while the rest would go to solid waste landfills. Currently, the United States EPA (U.S. EPA) does not consider LIBs to be hazardous based on TCLP; rather, they are hazardous due to their flammability but are managed as a Universal Waste. In contrast, California characterizes LIBs as hazardous due to cobalt levels that exceed California metal toxicity thresholds.
Relevant environmental and health impacts for the disposal of the carbonaceous elements, along with the non-metals, must be considered. For example, the graphite present in the EV battery waste stream represents a quarter of the entire waste stream, and the effects of particulate carbon on the respiratory system and pulmonary function are well-documented (NIOSH, 2007). The electrolytes, binders, and solvents are also problematic. For example, the electrolyte salt LiPF6 forms hydrogen fluoride gas in the presence of water or moist air, which is severely toxic and is a listed hazardous waste (U-134). While in one experiment, none of the standard LIB metals (aluminum, copper, lithium, manganese, steel, cobalt, and nickel) have failed the U.S. EPA TCLP in experiments run (Richa) using a specific formulation of LIB (LiAlNiCoMnO2 from Panasonic), a preliminary analysis suggests that the metal concentration in LIB leachate could exceed US Primary and Secondary Drinking Water Standards (U.S. EPA,2009), the EU Drinking Water Directive (EU, 1998) as well as the World Health Organization’s guideline limits (WHO, 2008). The introduction of EV LIBs to solid waste landfills may introduce risks due to, as stated above, leakage of electrolytes, presence of heavy metals (e.g., copper and nickel), reactive lithium salts (e.g., LiPF6), and large quantities of carbonaceous materials.
Regulatory Oversight of the Management of End-of-Life Rechargeable Batteries in Electric Vehicles: Federal (U.S. EPA)
The federal Universal Waste regulations were adopted in 1995 (FR Doc. 95-11143) and are found in Title 40 of the Code of Federal Regulations (CFR) in part 273. They apply to four types of universal waste, namely batteries, pesticides, mercury-containing equipment, and lamps. There are also four types of regulated entities (“participants”) in the universal waste system, namely small quantity handlers of universal waste (SQHUWs), large quantity handlers of universal waste (LQHUWs), universal waste transporters, and universal waste destination facilities.
The U.S. EPA considers batteries (including LIBs) to be a universal waste; batteries are defined in § 273.9. The definition of battery in the final rule is ‘‘a device consisting of one or more electrically connected electrochemical cells which is designed to receive, store, and deliver electric energy. An electrochemical cell is a system consisting of an anode, cathode, and an electrolyte, plus such connections (electrical and mechanical) as may be needed to allow the cell to deliver or receive electrical energy. The term battery also includes an intact, unbroken battery from which the electrolyte has been removed.’’ Batteries may not be crushed or broken to remove the electrolyte.
The applicability of the Universal Waste regulations to batteries is found in § 273.2. Specifically, § 273.2(b)(3) dictates that a battery, as defined in §273.9, must exhibit a characteristic of a hazardous waste (found in § 261 Subpart C), in order to be covered under § 273 as a universal waste. While lithium-ion batteries contain less toxic metals (e.g., lead or cadmium) than other types of batteries (e.g., lead acid batteries), they can be a safety hazard as they may contain flammable electrolytes (see the 2016 Samsung Galaxy Note 7 recall for battery fires). As such, lithium-ion batteries may be considered a hazardous waste under § 261.21(a)(2); thus, the EPA Hazardous Waste Number for lithium-ion batteries would be D001.
Footnote: U.S. EPA has identified two categories of hazardous waste – listed and characteristic. Listed wastes are determined by generators, as there are no approved test methods for their determination. Characteristic wastes are so named because they exhibit specific characteristics under 40 CFR 261, Subpart C. These are ignitability (waste code D001); corrosivity (waste code D002); reactivity (waste code D003); and toxicity (waste code D004 – D043).
The shipment of live and/or discharged lithium-ion batteries is regulated by the U.S. Department of Transportation (DOT) in 49 CFR, paragraph 173.185(j). The Pipeline and Hazardous Materials Safety Administration (PHMSA) is a sub-agency of the DOT which is responsible for publishing the applicable transport regulations applicable to (but not limited to) batteries.
As a general summary for how waste batteries are managed under the universal waste rules, any universal waste battery that shows evidence of leakage, spillage, or damage that could cause leakage must be contained. The container must be closed, structurally sound, and compatible with the batteries. Batteries or battery packs may be sorted, mixed, discharged, regenerated, disassembled into individual batteries, or removed from products as long as the individual battery cell is not breached. Cells may be opened to remove electrolyte from the battery but must be closed again immediately. Electrolyte or any other material generated by the handler must be evaluated to determine if it is a hazardous waste and, if so, managed appropriately as a RCRA hazardous waste.
Regulatory Oversight of the Management of End-of-Life Rechargeable Batteries in Electric Vehicles: DTSC
22 California Code of Regulations Division 4.5 Chapter 23. LIBs are considered a Universal Waste under California law. The requirements for Universal Waste management in California generally mirror those of the federal government; the “Mercury-Containing and Rechargeable Battery Management Act” of 1996 mandates that batteries be managed by each state at least to the level noted in 40 CFR part 273.
Regulatory Oversight of the Management of End-of-Life Rechargeable Batteries in Electric Vehicles: CalRecycle
CalRecycle’s purpose is to protect the environment and preserve resources by empowering Californians to reduce, reuse, and recycle. CalRecycle manages numerous recycling programs, including the Covered Electronic Waste Consumer Fee and Payment Program and various Extended Producer Responsibility Programs.
CalRecycle supports recycling and market development of recovered materials through various incentives such as grants, loans, and technical assistance.
CalRecycle also coordinates with the Department of Toxic Substances Control on hazardous waste and universal waste issues.
Regulatory Oversight of the Management of End-of-Life Rechargeable Batteries in Electric Vehicles: European Union
EU Battery Directive (2006/66/EC). The Batteries Directive is the only piece of EU legislation that is entirely dedicated to batteries. Its provisions address the lifecycle of batteries, i.e. design, placing on the market, end-of-life (EOL), collection, treatment and the recycling of spent
batteries. It defines objectives, sets targets and outputs, identifies measures to meet them and establishes additional provisions to enable and complete these key requirements. The Directive applies to ALL batteries; it classifies LIBs for EVs as “industrial batteries”. The Directive’s primary objective is to minimize the negative impact of batteries and waste batteries on the environment to help protect, preserve and improve the quality of the environment. It also aims to ensure the smooth functioning of the internal market and avoid the distortion of competition within the EU. Finally, it links the environmental impacts of batteries to the materials that they contain; if spent LIBs are landfilled, incinerated, or improperly disposed of at the EOL, then their constituent substances can enter the environment. Some requirements/prohibitions in the EU Directive include labelling requirements (Articles 20 and 21), requirement of treatment and recycling of ALL collected spent batteries (Article 12.1.b), and the mandate that producers of industrial batteries (i.e., LIBs in EVs) do not refuse to take back waste industrial batteries from end-users.
There are concerns about some processes involved in the recycling of LIBs. For example, the pyrometallurgical process used to extract metals from LIBs results in high amounts of greenhouse gas emissions; this may be partially compensated for by the amount of lithium, cobalt, copper, and nickel recovered by the process, and the resulting nullification of the need to extract said metals in the first place.
The Directive obligates metal shredding facilities to remove batteries before recycling/shredding operations; otherwise they may be sources of dust and heavy metal emissions. These include cobalt, nickel, or manganese. Lithium hexafluorophosphate (LiFP6) is an electrolyte that is also possibly hazardous (as stated previously, can form toxic hydrogen fluoride gas if exposed to water) (Lebedeva 2016).
Also note – the hazardous waste classification status of LIBs is not necessarily uniform throughout the EU. The waste designation situation of batteries for re-use is also unclear; batteries that are prepared for, but not currently being reused after their initial 10-year lifespan in an EV could be considered waste, while those that have begun to be reused are not waste. Furthermore, the extended producer responsibility (EPR) issue has yet to be addressed, as in the current Directive, the producer that first placed the LIB on the market would be ultimately responsible for the eventual scrapping/recycling of the battery, regardless of the number of secondary uses it may have had.
Regulatory Oversight of the Management of End-of-Life Rechargeable Batteries in Electric Vehicles: United Kingdom
LIBs are considered dangerous for the purposes of the Carriage of Dangerous Goods and Use of Transportable Pressure Equipment Regulations 2009. There is also the Waste Batteries and Accumulators Regulations of 2009 (2009 No. 890), which partially implement EU Directive 2006/66/EC. The Directive bans the disposal of waste industrial batteries (e.g., Lithium-ion) via
landfill or incineration. LIBs in EVs are considered a hazardous waste in the UK. EPR mandates apply as in the EU Directive (see previous section).
Regulatory Oversight of the Management of End-of-Life Rechargeable Batteries in Electric Vehicles: Japan
Japan passed The Act/Law on the Promotion of Effective Utilization of Resources in 2001, in which LIBs are considered as “specified resource-recycled products”; producers are required to promote self-collection and recycling. The Act/Law employs the idea of EPR. The national government has a responsibility under the regulations to establish standards for labeling of batteries, in which the labels should prominently display the material/chemical composition for sealed batteries, in order to facilitate proper self-collection and recycling. Compliance “points” are the design, size, and prominence of the identification marking. Batteries must be sorted by type. Battery manufacturers, as well as the manufacturers of products (such as EVs) using LIBs must disclose information every fiscal year to the Japanese government regarding metrics for the self-collection and recycling of waste sealed batteries that they conducted individually or collectively.
There was an incentive in the creation of the regulations to promote new battery design that is easier to take apart; as of this date, this has not come to fruition. It is unclear if LIBs from EVs are considered a hazardous waste in Japan.
(Tasaki 2014)
Regulatory Oversight of the Management of End-of-Life Rechargeable Batteries in Electric Vehicles: China
In China, the technical policy for pollution control on waste battery, published in October 2003, is the first law or regulation especially for waste batteries. It regulated the pollution prevention and control of the whole recycling process of waste batteries and set up guidelines and basic principles for the recycling and resource utilization of waste batteries.
In 2006, National Development and Reform Commission, Ministry of Science and Technology, and Ministry of Environmental Protection of the People’s Republic of China put forward the auto product recovery and usage technology policy, which instituted a form of EPR for EV manufacturers to take responsibility for the LIBs in their vehicles.
In 2016, the technology policy for the recycling of power battery regulated EV manufacturers, LIB manufacturers, and “cascaded use enterprises” of waste EV LIBs.
It is unclear if China currently has regulations in place regarding the final landfill/disposal of EV LIBs after the valuable metals have been removed, nor for how to manage the waste EV batteries during their treatment to prevent the release of toxic/hazardous substituents, such as LiFP6/HF or particulate carbon matter.
China’s Ministry of Industry and Information Technology (MIIT) published national guidance in early 2018 that automakers bear the responsibility for recycling and disposal of electric vehicle batteries. Additionally, the guidelines push automakers to standardize batteries and design easy to disassemble products in order to address the costly manual disassembly. (Interim Measure for the Management of Recycling and Utilization of New Energy Vehicle Power Battery, China regulations)
(Xu 2017)
Related News Articles
Forbes: The Clock is Ticking on Electric Car Batteries – And How Long They Will Last, Sept 30, 2019
Engineering.com: Will Your Electric Car Save the World or Wreck It?
References
1. Frost & Sullivan. (2014). 2020 Vision: Global Lithium-Ion Battery Market. Frost & Sullivan, San Antonio.
2. Richa, Kirti, “Sustainable management of lithium-ion batteries after use in electric vehicles” (2016). Thesis. Rochester Institute of Technology.
3. M. Lowe, S. Tokuoka, T. Trigg, and G. Gereffi, “Lithium-ion Batteries for Electric Vehicles : The U.S. Value Chain,” CCGC, Duke University, Raleigh, North Carolina, October 5, 2010.
4. EIA. (2012). Annual Energy Outlook 2012. Light-duty vehicle sales by technology – United States. http://www.eia.gov/forecasts/archive/aeo12/
5. Nishino, Hiroshi. (2010). Key Technology for EVs; Lithium-Ion Secondary Battery.
6. Chaitanya K. Narula et al., FINAL REPORT: ECONOMIC ANALYSIS OF DEPLOYING USED BATTERIES IN POWER SYSTEMS 2-3 (June 2011).
7. Stringer, David and Jie Ma. “Where 3 Million Electric Vehicle Batteries Will Go When They Retire.” Bloomberg, June 27, 2018.
8. ecoinvent Centre. (2010). ecoinvent data and reports v.2.2. Dübendorf, Switzerland: Swiss Center for Life Cycle Inventories.
9. Lebedeva, N and Brett, L (2016), Considerations on the Chemical Toxicity of Contemporary Li-Ion Battery Electrolytes and Their Components, J. Electrochem. Soc. 2016 volume 163, issue 6.
10. Tomohiro Tasaki. 2014. The Recycling Scheme for Compact Rechargeable Batteries in Japan – under the Act on the Promotion of Effective Utilization of Resources.
11. Xu, C., Zhang, W., He, W. et al. Environ Sci Pollut Res (2017) 24: 20825. https://doi.org/10.1007/s11356-017-9890-8

